
We offer custom manufacturing of Transcatheter Heart Valves from Design to Valve –TAVR, TMVR, Tricuspid, or Pulmonic Valves as per client’s IP
Learn More
We offer a wide array of consulting services to the medical device and structural heart valve industries.
Learn More
We manufacture GBR I bovine and porcine glut fixed tissue for transcatheter heart valve industry.
Learn More












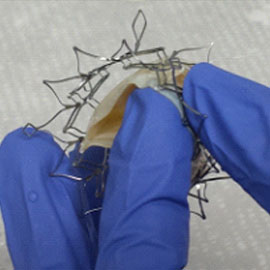
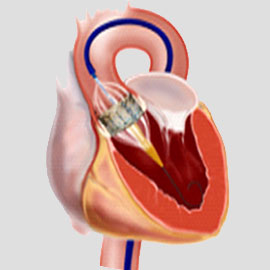
We contracted BridgeMed Solutions to work on a tissue treatment for our Transcatheter Aortic Valve Replacement (TAVR) technology. Due to their deep knowledge and experience in this field, they have done an excellent job in keeping the project timeline and budget on track. A key factor is that they spend the time to understand our wants and needs. Thanks to BridgeMed’s help the project
is progressing very smoothly and as planned.
We’re pleased with the work performed by BridgeMed on our Mitral Valve product. From the very beginning we knew that we have made right choice in trusting BridgeMed to assist with our valve. The expertise and the experience they bring to the table in transcatheter valve replacement therapies are unmatched.
We had our chemical (wet) lab setup from Bridgemed for cross-linking our tissue to be used in our Transcatheter Aortic Valve (TAVR) leaflets. We’ve to say Bridgemed is very hands-on, there is either the learning curve or learning on client’s “dime” – they knew the work thoroughly. They were able to successfully implement a wet lab setup at our facility. Also they worked with us on many consulting assignments with our TAVR valve