CEPI was born from the encounter between the vision of an engineer and a manufacturer. From these roots, it has grown into an idea factory that puts technology squarely at the center of our work. With the high levels of customization going into our projects and the diversity of needs we intercept across the globe, our research is always ongoing and has translated into unparalleled technological range.
Our motto: if a process needs it, we can do it. If we can’t do it yet, we will develop it. Our vision prioritizes flexibility, research, and customization and our mission is to build installations that cover the entire production from storing to dosage. Because of this, and with the flexible approach that sets us apart, we innovated pretty much all aspects of our field, matching general market trends and specific needs reported by our sales team, agents network, project managers, engineers, software designers and the commissioning teams themselves. We consider the installation our product and customization our service.
Our R&D team itself aggregates engineering, manufacturing and food technology expertise. We monitor scientific and technological developments as well as feedback from markets and installations, and transform it into new tools. Not just for materials and processes, but with an important investment in hygiene and safety concerns, as well as sustainability and the preservation of food properties. The effort to design installations and technologies that optimize energy and material consumptions creates a constant and welcome demand for new solutions and customized developments.
Every installation is different, and every story is important. Our work would not be possible between the kind of ongoing dialogue we have established not just with customers, but between all the teams in our crew. Adaptations and inventions happen pretty much at every stage, adding to the shared knowledge that informs all the work we do. We bring to the table a global knowledge of materials, environments and markets as well as the continued research that goes into developing around 300 unique systems every year. We thrive on the challenge.
Globality, flexibility, expertise and dialogue: this is the special recipe behind a rich history of developments that includes too many technologies to list comprehensively. Let’s name only a few of the most important ones: the equipotentiality technology in our fabric silos, the cold dosing of fats, the low energy impact system for the progressive cooling of flours, our exclusive welding technique to prevent contamination, our scrap rework systems and all the options for microingredient dosing that make our stations truly suitable to all materials, including the more challenging ones such as salt and sugar.

Cold dosing of fats: stabilizes temperatures and preserves the organoleptic characteristics of ingredients such as fat, margarine and butter
Our automation systems are completely integrated and customized, finely optimized during testing and launch. We have developed many different tools including native PLC and software to match very diverse production lines, and extending the power of our traceability system to include complete virtualization of data and total information exchange. We are integrating each new mobile HMI device as well as the automatic management of all utilities.
Our new home will be coming next spring, and it has been designed to include a testing and prototyping room with 240 m2 metrology room. This is the measure of how important we consider our research. In the new test room we will be able to carry out deep mixing, dosing and scrap recovery tests. We are not just building new machines: we are calibrating our work to what the final product needs, through a collaborative effort that aims to share and build together, and support our partners’ own research.
The latest developments
Despite the harsh times we went through, 2019 and 2020 were fertile for innovation in CEPI. We are looking ahead at the future and working to create the conditions to support it, any way we can.
The 3 in 1 is the latest development in our technology: a station that weighs, filters and blends in a single unit. It comes with vertical blender for the production of premix that optimizes mixing and production times and ensures the highest flexibility across a wide range of food sectors. Fast, accurate and clean, 3 in 1 delivers a homogeneous mix of powders even for quantities lower than 1% in the span of 3-5 minutes.
The Trevibox silo is the latest addition to our solutions for the indoor storing of macro ingredients. An antistatic Trevira fabric silo equipped with a fluidized bed, Trevibox combines the advantages of Trevira fabric (customizability, antistatic properties, balance between environment and product) with fluidization to deliver flexibility, safety, resource efficiency and high product quality through flour oxygenation.
We increased the cooling power of our flour cooling system, which can automatically and independently take the flour to the temperature required for further processing and remove the need for cooling agents and manual intervention. Our system not only ensures stability, precision, and homogeneity in the dough but is a low impact technology that minimizes energy consumptions.
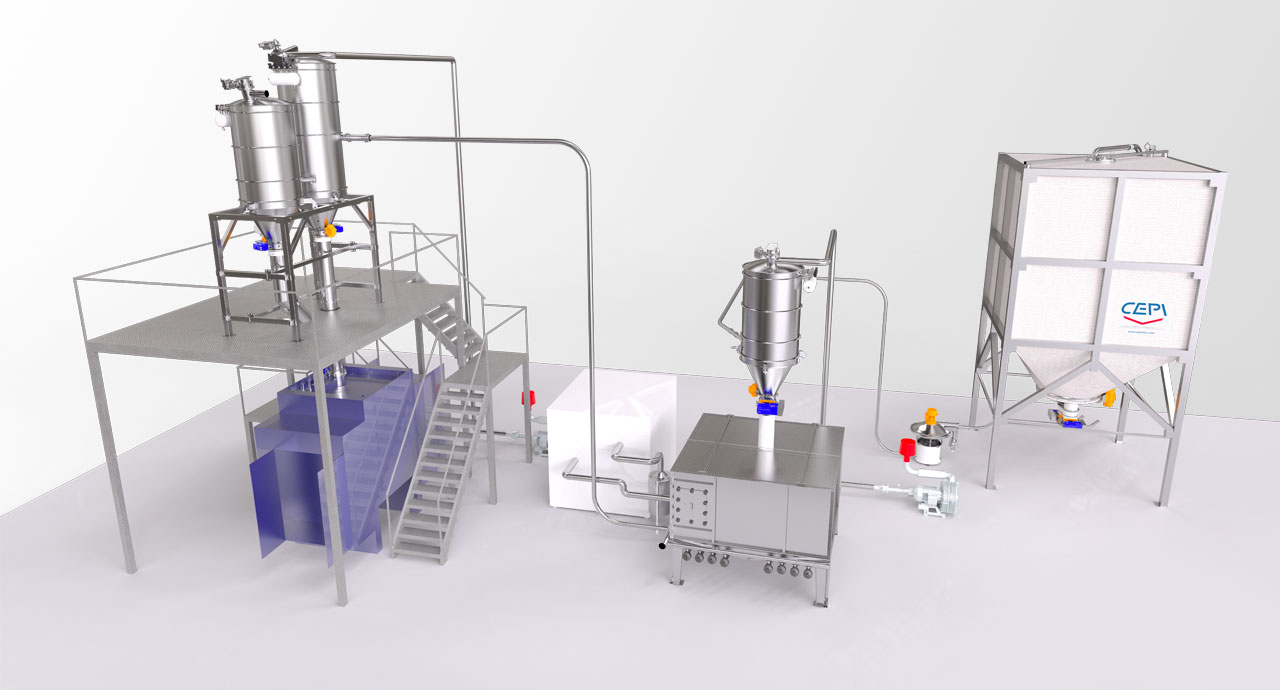 Flour cooling system: high stability and low energetic impact
Flour cooling system: high stability and low energetic impact
Trimix is a broad spectrum metering station for micro ingredients with a capacity up to 2500 kg per hour. It can now be combined with hoppers of all sizes, achieving higher speed and volume, and is able to handle a wide range of materials including challenging ones such as lecithins. Trimix is suitable for continous dosing and comes with fixed or mobile scale. It has an easy-to-clean design with air blade system to clean the bearings.
Safety and hygiene are important drivers to our research. Our in-house produced slide valve is ATEX conform and protects against explosions by rapidly insulating the container. It can be installed on pneumatic conveyance both by suction and pressure and is suitable for all powders, including abrasive ones. We are in the process of finalizing new technologies for the hot cleaning of silos that protect operators and ensure a low environmental impact by removing pesticides and chemicals from the process.
Get in touch at booth 2017 in the West Hall for more information about our research and technologies. We are eager to share our developments with you – and create new ones to suit your process!